
Lead acid batteries are one of the most commonly used batteries for vehicles and inverter use.
Besides this they are also used in solar applications, ups and other areas.
They have a very low energy to weight ratio but they have a longer life and less cost as compared to many of their counterparts. Many of the lead acid battery manufacturers keep innovating to get the best design for optimum performance.
What are lead acid batteries
Lead acid batteries are a type of rechargeable battery and they were invented by physicist Gaston Plantae and are one of the first rechargeable batteries ever created.
Despite being low energy density they can still deliver high surges of current which are typically needed by the starter batteries.
When compared to the latter technologies they come up as a convenient and inexpensive choice to meet power needs.
Structure of lead acid batteries
The lead acid batteries consist of two electrodes which are in the form of plates. The negative electrode is made up of porous lead and the positive electrode of lead oxide. These both electrodes are submerged in electrolyte which is a solution of sulfuric acid and water. Separators keep the electrode plates from sticking on each other and prevent short circuiting.
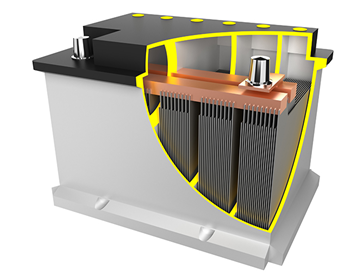
Electrical energy is stored inside the lead acid battery because of the reversible reaction that is happening inside it.
The reaction uses sulfate from the sulfuric acid for the formation of sulfate crystals and this leads to gradual concentrating of the electrolyte and this is the reason that water needs to be added to it.
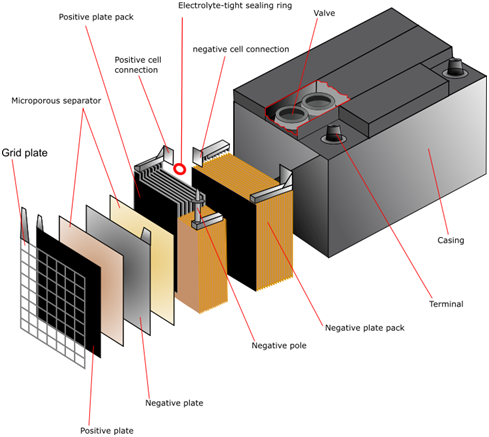
1.Plate:
As mentioned earlier the plates of negative and positive electrodes are dipped in the electrolytic solution of sulfuric acid. The negative and positive electrodes have the active material pasted on grids. A number of alternate negative and positive plates are arranged inside the battery case. A plate has a grid on which the material is pasted.
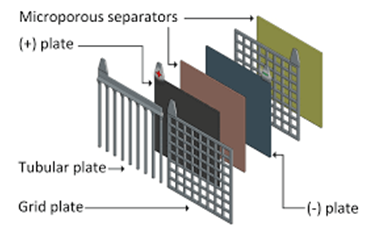
The grid:
The grid is usually made of lead with a bit of additives to strengthen it and improve electricity flow. Addition of antimony helps them to carry more amount of pasted material which helps the plates to be thicker thereby increasing the battery life. Using grids of alloys rather than pure lead helps to garner more mechanical strength and low self discharge.
Using lead-calcium alloys helps to have a lower self discharge and less frequent watering of the battery. Some make the grids using lead-selenium also. Different battery manufacturers innovate and come with the best and optimum design to make the batteries.
Active material:
The material which is actively involved in the chemical reaction to produce electrical energy is known as the active material. Here the porous lead in the negative electrode, lead oxide in the positive electrode and the electrolyte are the active materias.
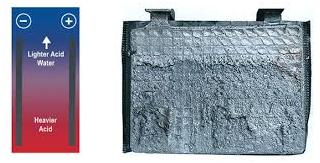
The active material is pasted on to the surface of the grid in the respective positive and negative electrodes. The chemical composition is unique with regards to each battery manufacturer.
Due to multiple charging and discharging cycles the paste on the grid degrades and it gets shed into the bottom of the battery.
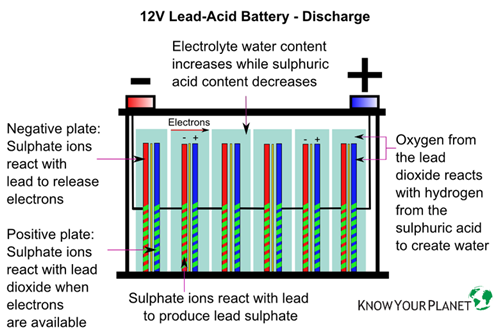
2.Electrolyte
The electrolyte used is a mixture of sulfuric acid and water. During the charging process the sulfuric acid undergoes reaction with the lead in both the electrodes to produce lead sulfate. When the battery is discharging, the reverse happens.

3.Case
The plates once made and dried are inserted and placed inside the container made of plastic. The material used to form the outer casing or container should be non-reactive and should carry the contents inside properly. They are designed with ribs at the bottom on which the positive and negative electrodes rest. The containers should be sturdy enough to handle transportation and frequent handling.

4.Separators
The separators as the name suggests keep the negative and positive plate separated and thus prevents short circuiting and shedding of the material. The separators should be porous, permeable and have ionic conductivity. These days separators made of PVC and polythene are used commonly.
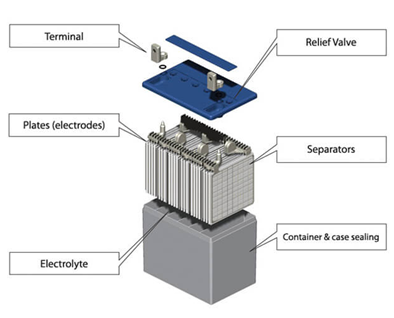
Types of lead acid batteries
1.Depending on the type of usage:

(a)Starter batteries:
Starter batteries are used mostly in the vehicles for starting, lighting and igniting them, hence they are also called SLI batteries.
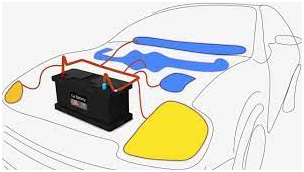
They have thinner plates to increase the surface area for reaction and produce a great amount of current for a short span of time to get the engine started.
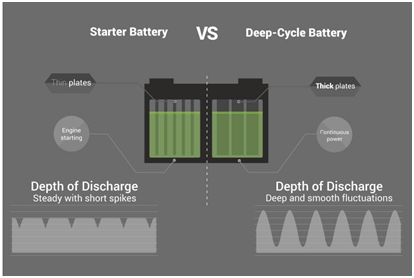
(b)Deep cycle batteries:
Deep cycle batteries are meant to get discharged of most of their capacity and they provide long hours of power consistently.
2.Depending on type of design:
(a)Flooded:
Based on the design,these are the most commonly used batteries in the battery industry. Going by their name, they are called flooded because of the sulfuric acid inside them which is free flowing and hence the batteries have to be taken care so as not to spill or leak it.

They are less expensive as compared to the valve regulated ones.
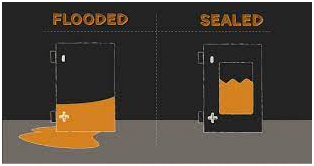
(b)Sealed type :
The Sealed type lead acid battery is also called the VRLA or Valve Regulated Lead Acid battery. Here the electrolyte is not free flowing rather it is present either as in a gel form or absorbed on to a glass mat.
The main two types of sealed lead acid battery are:
(1)Absorbent glass mat
(2)Gel Battery
The sealed type battery prevents the leakage and spilling of the electrolyte.
Summary:
Lead acid batteries are a convenient and inexpensive way to store energy and have been used since a long time.
If you want to buy lead acid batteries, then go for Sarex, which offers inverter and E-rickshaw batteries and is a trusted lead acid battery manufacturer. Visit their website at www.sarexindia.com and explore their wide range.
